Integrity testing is a critical quality assurance process when using hydrophilic membrane filter cartridges in applications requiring bioburden control. Most manufacturers, including Graver Technologies, conduct an integrity test on each universal segment and/or finished cartridge prior to release to provide assurance that the cartridge will perform as required. This integrity test, typically either a diffusion test or bubble point, is performed on an integrity test system in the manufacturing area with the values recorded as part of the quality program. This ensures the end user that the product they receive will conform to the retention requirements. Nonetheless, it is
common practice for the end user to integrity test again, prior to using the cartridges in the application. Occasionally, end users report integrity failures at this stage. But upon further examination these are usually found to be false failures, with the most common culprit being the failure to adequately pre-wet the membrane. Other potential causes are damaged or missing o-rings, incorrect values being utilized on the test equipment or leaks in the test system itself. The least likely cause for integrity failures at this stage is a non-integral cartridge!
A typical membrane cartridge will have anywhere between 6 ft 2 and 8 ft 2 of surface area per 10” filter length. Assuming a media, such as polyethersulfone (PES), is 70% porous, the filter then has about 6 – 8 trillion pores, all of which must be filled with liquid in order to conduct a valid integrity test and obtain a passing integrity test value. Therefore the pre-wetting process is extremely critical and the success depends upon many factors that include:
- Characteristics of the wetting fluid (alcohol, water, temperature)
- Characteristics of the membrane (hydrophobic, hydrophilic, material of construction, pore rating)
- Process conditions (time, flow, pressure)
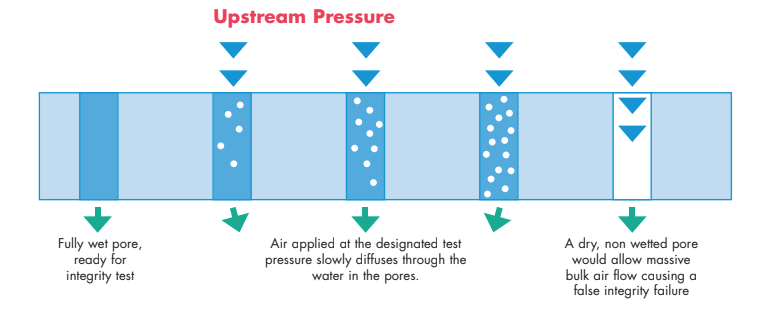
The process used to wet one membrane type may be quite different than another type (PES versus nylon for instance) and thus the manufacturer’s guideline should be followed to minimize the risk of failure. Even chemically similar membrane types may vary from manufacturer to manufacturer due to slight chemical differences in the membrane. For instance, in the manufacturing of PES membranes, various amounts and types of wetting agents may be added to improve the wettability of a membrane. Wetting agents, such a polyvinylpyrrolidone (PVP), will improve wettability but may extract out, and while posing no issue for food and beverage or pharmaceutical application, may become an extractable concern for microelectronics applications where measures of extractables are at the parts per trillion level.
Improving the Pre-wetting Process
The general pre-wetting guideline for the Graver ZTEC product line is to flow at 10 GPM per 10” for 20 minutes. For some customers, however, limitations in pump capacity or availability of the wetting fluid may make following these guidelines difficult. In addition, while the bulk of the pores are easily wetted, some areas of the cartridge, such as along the end cap/media interface tend to be more difficult. Therefore we offer a number of steps that can be taken to facilitate the pre-wetting process.
-
Increase the temperature of the wetting fluid - Increase the temperature above ambient, even as high as the typical sanitization temperature of 176°F (80°C). Caution must be exercised in controlling differential pressure to under 10-15 psid to avoid damage to the filter.
- Introduce back pressure – Closing the downstream valve will cause back pressure with the goal to deliver the wetting fluid at a high pressure to improve distribution of the flow throughout the media, particularly along the end cap/media interface. This is particularly useful if there are pump capacity issues.
- Increase flow through the cartridge – increasing the flow above the recommended 10 GPM/10” for 20 minutes ensures distribution through the cartridge. As in any high flow situation, differential pressure should be maintained well below the recommended maximum (see product data sheet).
- Increasing the pre-wet time. If time permits, soak the filter overnight or recirculate the wetting fluid for an extended period of time.
- Use of a wetting agent – Some manufacturers offer filters with a food grade/pharmaceutical grade wetting agent such as glycerine. Graver offers this in the ZTEC product line as the QW (Quick Wet) option.
- Use of alternative wetting fluids – other fluid such as alcohol, either isopropyl or an ethanol based alcohol (distilled spirits) will easily wet out a membrane.
Keep in mind, the least likely reason for an integrity failure is a non-integral cartridge. An indication of an improperly pre-wet filter is an improved integrity test value upon subsequent retest.
Published Values
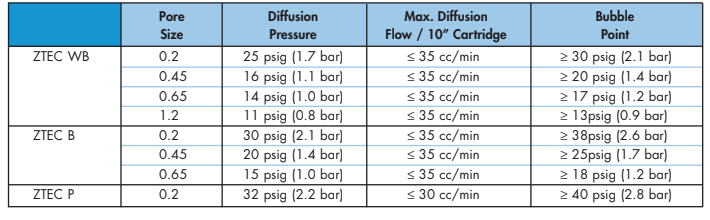
Selection of the proper integrity test value is critical. The values for the Graver Technologies membrane products are printed on the product data sheet, the Qualification or Validation Guide as well as the product insert that comes with the product.
This technical brief was supplied by Graver Technologies. Browse our Graver product offering online here.
Updated